As a healthcare administrator, you know the importance of doing everything possible to ensure patient safety through the reduction of medical errors. HIPAA compliance, FDA approval and the  international ISBT-128 standard are significant factors in determining your safety policies and procedures. These organizations provide guidelines for blood and IV bag identification labels and managing your healthcare service’s inventory.
international ISBT-128 standard are significant factors in determining your safety policies and procedures. These organizations provide guidelines for blood and IV bag identification labels and managing your healthcare service’s inventory.
RMS Omega’s specialists are available to review your operations and advise you on the best technology to use for blood and IV bag identification solutions. There are typically three key components:
- Printing software, either standalone or integrated into your blood bank information system
- Labels that are industry compliant and use FDA-approved adhesive
- Barcode printer that can integrate into the existing network and produce high-quality barcode labels
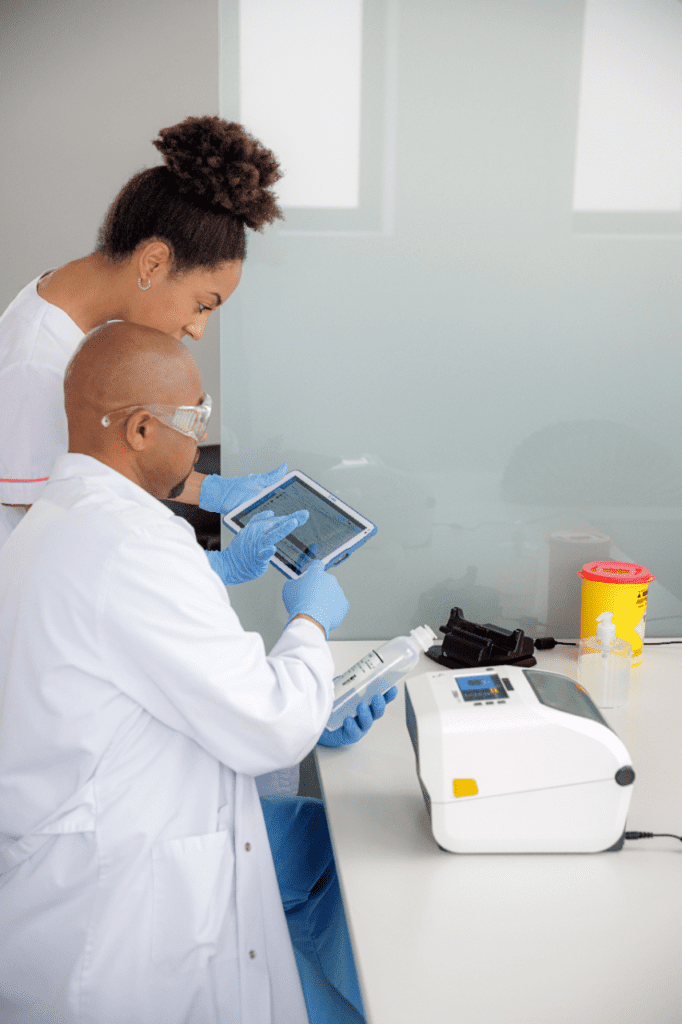
For over 25 years, we’ve developed partnerships with many reputable companies. This means the RMS Omega team can design and implement a full-service solution to your challenge of ensuring all blood and IV bags are identified accurately. Read on to learn more about a sampling of available products.
Full-Service Solutions for Blood and IV Bag Identification
At RMS Omega, we don’t just hand over an off-the-shelf package. Our specialists will work with you to identify needs specific to your healthcare operation. Only then can we suggest solutions and review the pros and cons of each option. Once you make a decision, we can work with you to make sure the implementation goes well, any training is done, and follow-up support is provided as needed.